
Okay, So first of all, does this oxygen fit my bonding preference According Thio Bond represent, Yes, it does. Let's do this with this oxygen right here.

So as long as you're bonding, preference agrees with what you see, that's going to be zero. Do you see that? But it also fits with my bonding preference. My equation says that formal charge equals group number, which is one minus sticks and thoughts, which is one. So it's gonna be one according to the equation. Remember that Bonding preferences say that hydrogen wants toe. So let's start off with the hydrogen even though we already know that this fits the bonding preference. What that means is I'm gonna be looking at group numbers and I'm gonna be looking at sticks and thoughts. Let's go ahead and do this example where I wanna look at each atom and I want to count the formal charges for all of them. There's usually not that many formal charges on the molecule. You're just looking at each Adam and saying, Does this have a formal charge? Does this have a formal charge? The net charges the collection of all of those sums together. You see it a little bit confused over like, Oh, does the formal charge go on the whole thing? Or is it just one, Adam? No. Now, this is an important point because I remember when I was an undergrad. The net charge is the term that we give for the sum of all the formal charges. So you take your group number, then you just subtract the sticks and the dots and you're good. A lot of times, you'll just be able to do this on your fingers.
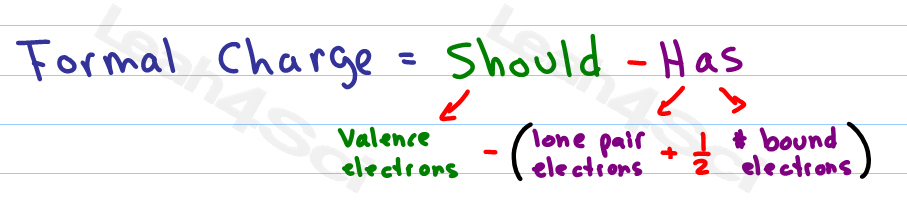
And then you subtract the valence electrons, which is just the sticks and the dots. So all you do to calculate formal charge is you take the group number, whatever that is, that could be Group four, Group five, whatever. So remember that the group number is how maney it wants the valence electrons, the sticks and the dots are the it actually has. Basically, a formal charges assigned whenever there's a difference between the number of Valence electrons and Adam wants toe have and the number of valence electrons it actually has. So let's go ahead and just jump right into it. And formal charges are just based on the entire idea of bonding preferences. So now that we understand bonding preferences so well, I want to move to a really related topic called formal charges.


 0 kommentar(er)
0 kommentar(er)
